Durable Relief For Your Chronic Rhinitis Patients
RhinAer® clinical outcomes

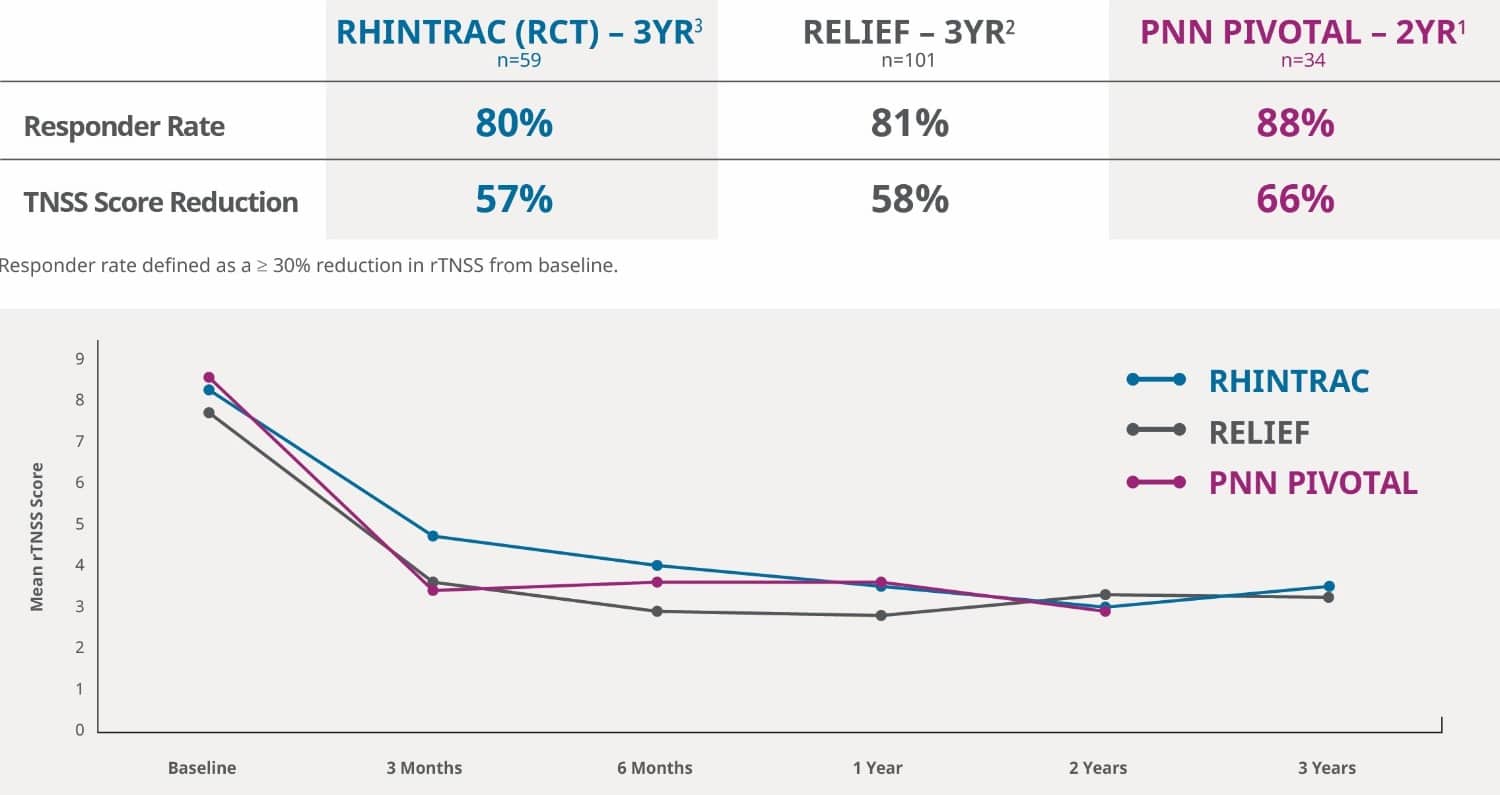
Lasting Relief with RhinAer
The RhinAer procedure is the most clinically studied non-invasive solution for chronic rhinitis. It has consistently established lasting and significant relief of chronic rhinitis symptoms in multiple clinical studies, with benefits sustained up to following a single RhinAer procedure. Three clinical studies demonstrated effectiveness in both allergic and non-allergic chronic rhinitis.1 Additionally, 3 years post post procedure, up to 80% of patients either reduced or discontinued their medications.2
Demostrated Effective, Long-Lasting Results
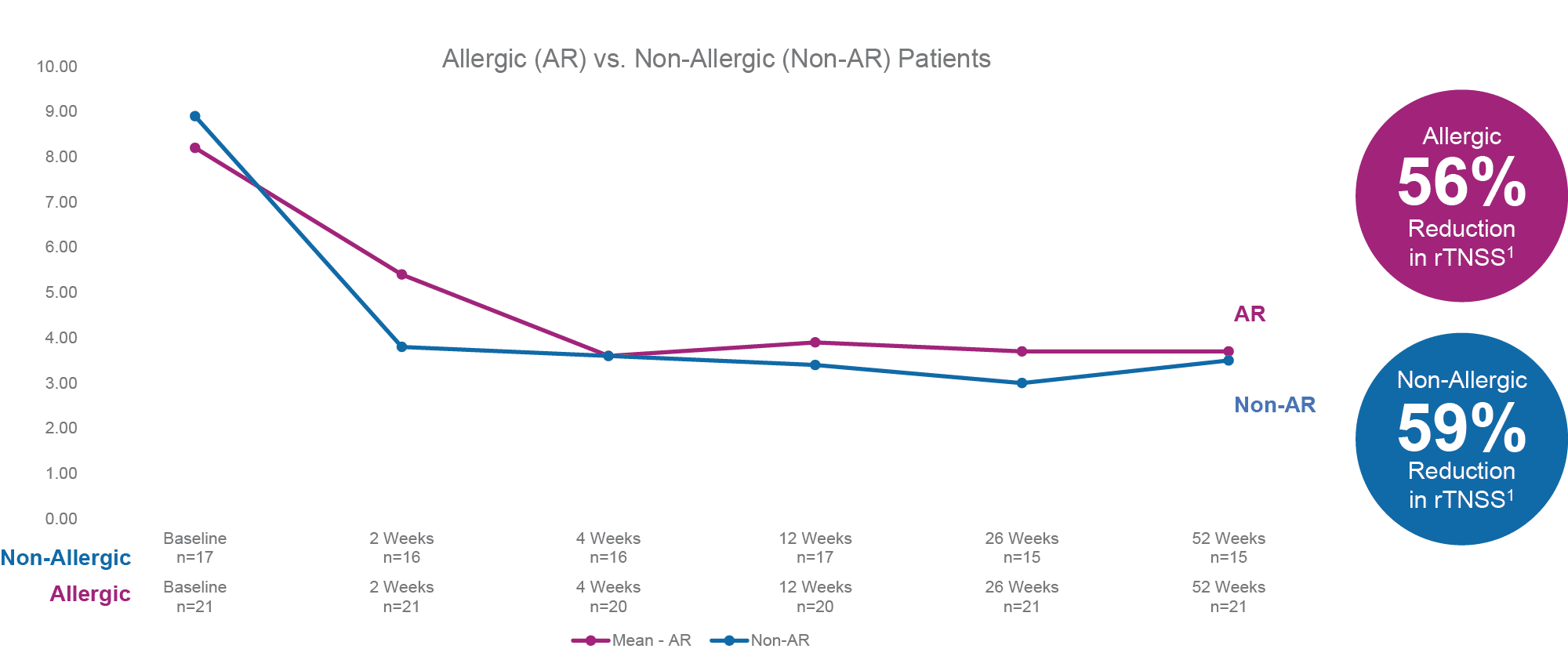
Relief From Both Allergic Rhinitis and Non-Allergic Rhinitis Symptoms
RhinAer Stylus provided significant symptom improvement in chronic rhinitis across allergic, non-allergic, and unknown etiologies. The RhinAer treatment showed comparable effectiveness in both allergic and non-allergic patients, achieving a 56% and 59% reduction in rTNSS respectively, sustained up to 1 year.1
High-level Evidence of an RCT Demonstrates Significant Improvements
The RHINTRAC Study is a prospective, single-blinded (patient), randomized controlled trial (RCT) with a sham procedure control arm. It yielded positive results affirming the durable treatment effect of the RhinAer procedure for chronic rhinitis. The study demonstrated significant symptom improvement and reduced medication usage maintained over 3 years.3
(Combined Active and Crossover Treatment Arms through 3 years)3
RhinAer Stylus achieved substantial and long-lasting reductions in CR symptons3
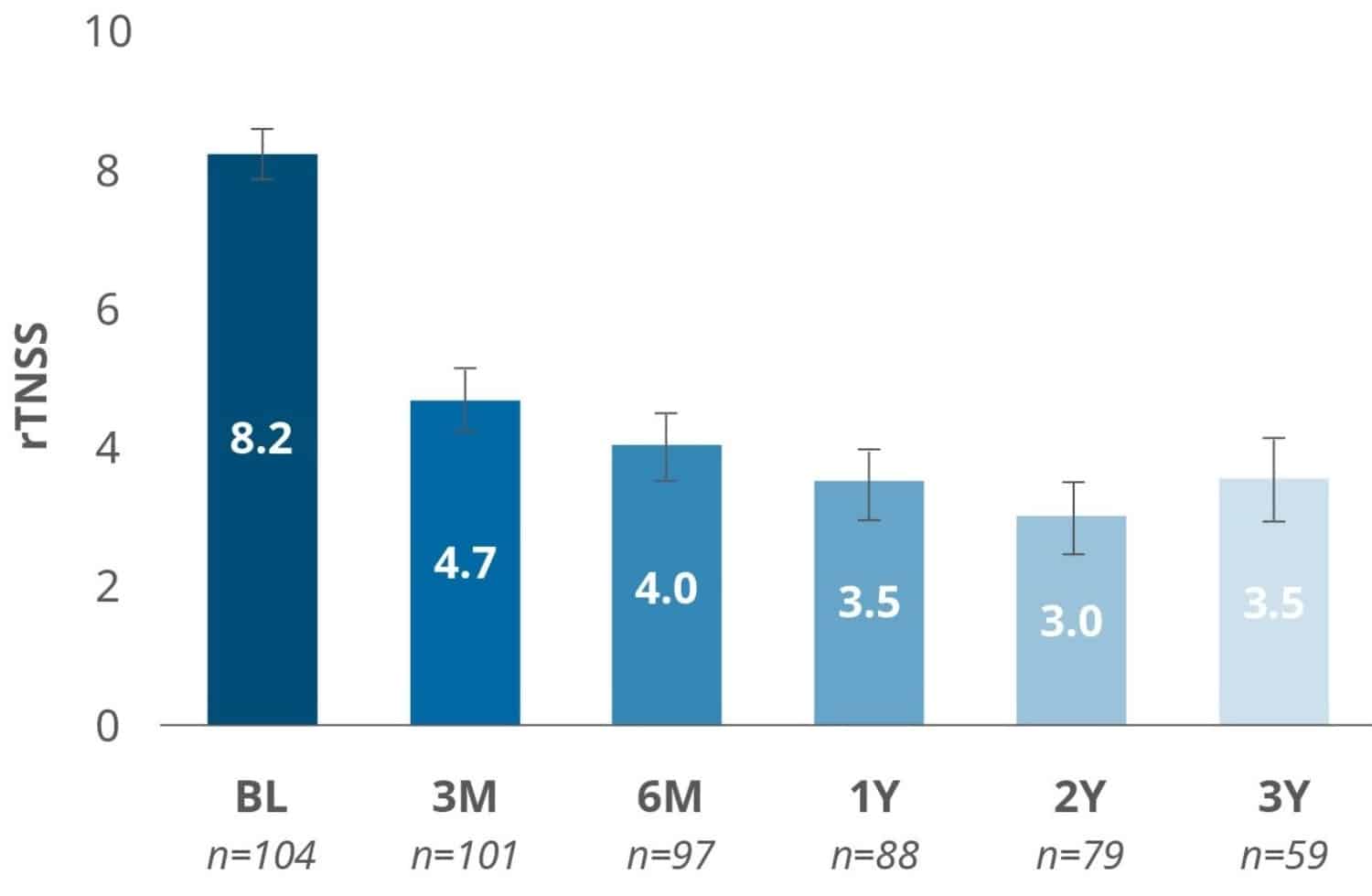
*P<.001 for TNSS comparisons to baseline for all timepoints and differences between active and sham arm responder rates
RhinAer Stylus Pivotal Trial
A prospective, muti-center, single-arm study was conducted to assess the safety and efficacy of RhinAer treatment targeting the PNN to address chronic rhinitis. The results indicate that the RhinAer treatment was well-tolerated and led to significant improvements in patient quality of life.1
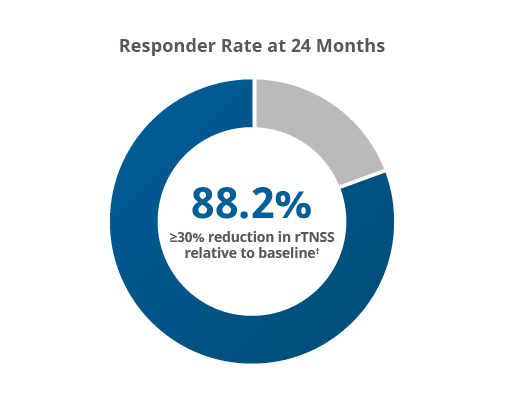
88.2% of patients were classified as responders after achieving ≥30% reduction in rTNSS compared to baseline.
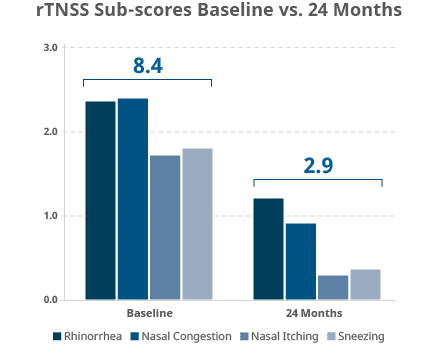
65.5% overall rTNSS reduction with all rTNSS sub-scores (rhinorrhea, nasal congestion, nasal itching, and sneezing) showing statistically significant (p<.001) reduction over baseline at 24 months.
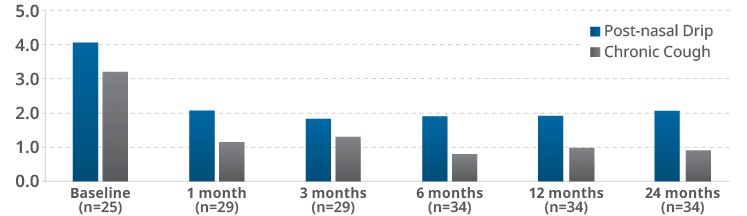
At 24-months post-nasal drip and chronic cough symptom scores remained significantly (p<.001) improved from baseline at all follow-up time points.
Study demonstrated that RhinAer Stylus significantly improves post-nasal drip (PND) and chronic cough1
“Chronic rhinitis treatment has been a longstanding challenge due to the limitations of medical therapy and the historical lack of minimally invasive, comprehensive procedure options. It is significant to add high-level evidence reinforcing the efficacy and durability of RhinAer Stylus, which provides a well-studied solution for those enduring the burdens of chronic rhinitis.”
Pablo Stolovitzky, M.D.,
Adjunct Assistant Professor, Department of Otolaryngology, Emory University; CEO of ENT of Georgia North, Atlanta; and principal investigator of the RHINTRAC trial.

WEB1323-27.B
Disclaimer
The RhinAer® Stylus is indicated for use in otorhinolaryngology (ENT) surgery for the destruction of soft tissue in the nasal airway, including in posterior nasal nerve regions in patients with chronic rhinitis.
Specific indications, contraindications, warnings, precautions, and safety information exist for these products. Aerin Medical relies on the physician to determine, assess and communicate to each patient all foreseeable risks of the procedure. A physician must always refer to the package insert, product label and/or instructions for use before using any of Aerin Medical’s products. Rx only.
References:
The Reflective Total Nose Symptom Score (rTNSS) is a validated patient reported outcome used to describe symptoms of rhinitis. The assessment consists of 4 nasal symptom domains (runny nose [rhinorrhea], itchy nose, sneezing, and stuffiness [nasal congestion]). Each item is rated from 0 (absent) to 3 (severe). The 4 domains are added together to provide an overall score ranging from 0 to 12.
- PIVOTAL STUDY: Ehmer D, McDuffie CM, McIntyre JB, Davis BM, Mehendale NH, Willis JH, Watkins JP, Kakarlapudi VV. Long-term Outcomes Following Temperature-Controlled Radiofrequency Neurolysis for the Treatment of Chronic Rhinitis. Allergy Rhinol (Providence). 2022 May 29;13:21526575221096045. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9158436/
- RELIEF STUDY: Lee JT, Abbas GM, Charous DD, et al. Three-Year Outcomes After Temperature-Controlled Radiofrequency Ablation of the Posterior Nasal Nerve for Chronic Rhinitis. American Journal of Rhinology & Allergy. 2025;0(0). doi:10.1177/19458924251360889
- RHINTRAC STUDY: Stolovitzky, J.P., Ow, R.A., Silvers, S.L., Tajudeen, B.A., McDuffie, C.M., Dean, M., Sedaghat, A.R., Phillips, K. and Takashima, M. (2025), 3-Year Outcomes of Temperature-Controlled Radiofrequency Ablation of the Posterior Nasal Nerve in Patients With Chronic Rhinitis. Int Forum Allergy Rhinol. e23577. https://doi.org/10.1002/alr.23577