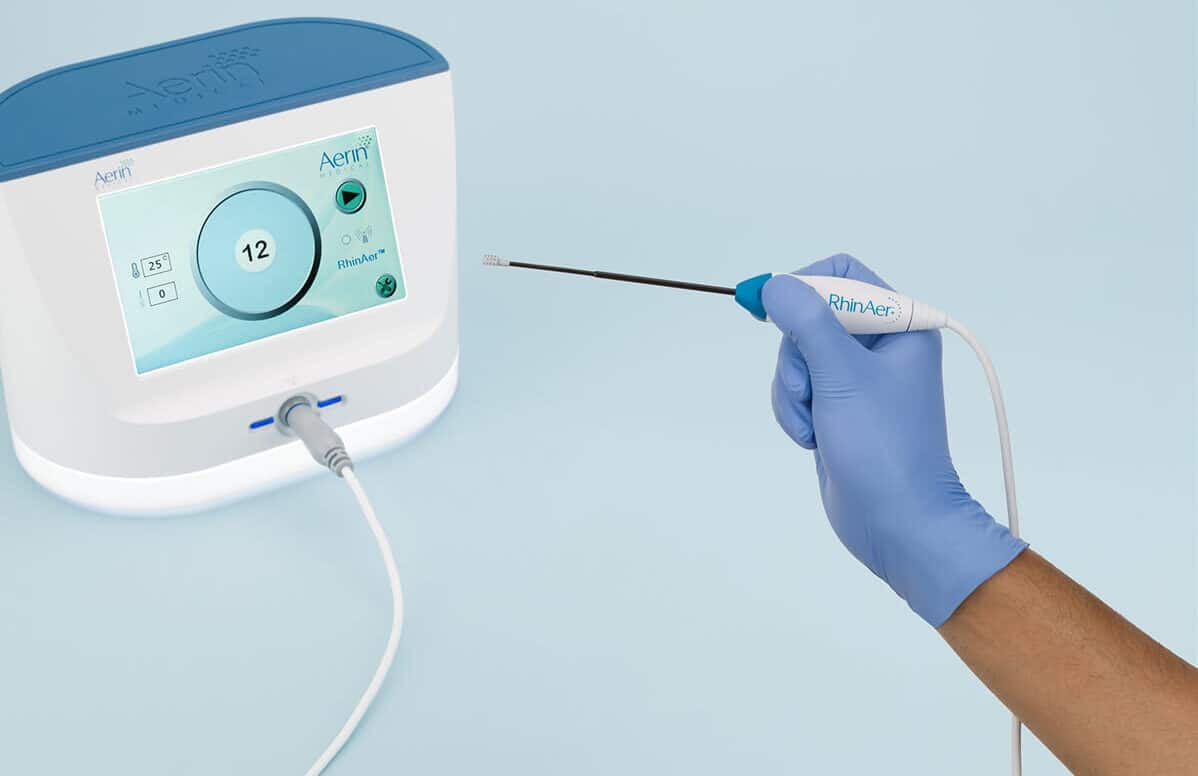
Introducing RhinAer+™ Stylus, The Next-Generation Device for Chronic Rhinitis Relief
Treat with Precision. Glide to Target. Trust the Outcomes.
Featuring temperature- and impedance-controlled radiofrequency, the RhinAer stylus is the only posterior nasal nerve (PNN) treatment that comprehensively targets multiple anatomic contributors to chronic rhinitis – providing proven, lasting relief of runny nose, congestion, and post-nasal drip. RhinAer+™ stylus builds on this proven technology with a next-generation design inspired by physician feedback.
RhinAer+ Stylus Now Features:
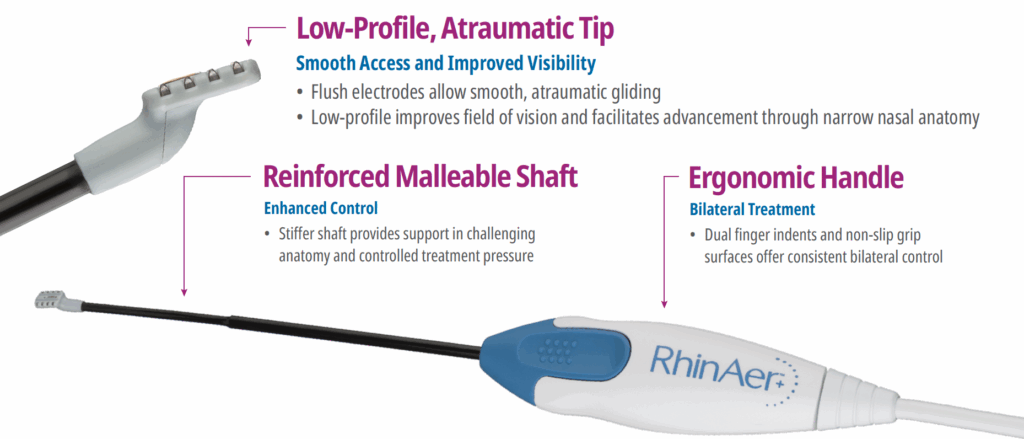
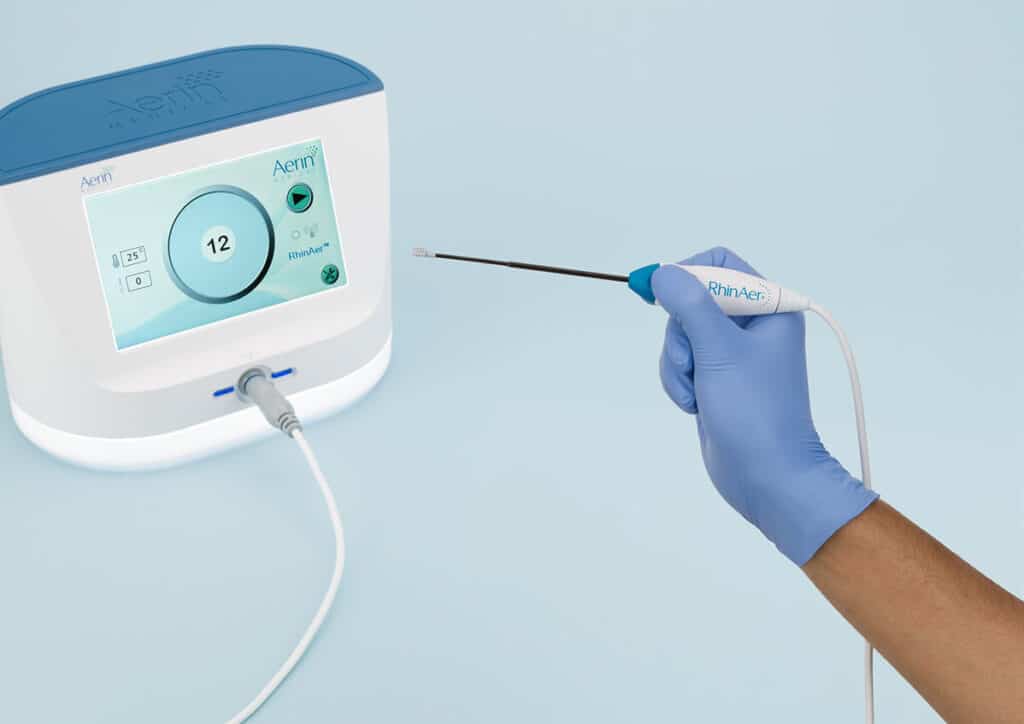
The RhinAer Stylus
For many patients with chronic rhinitis, medical management fails to provide the long-lasting relief they seek for their persistent runny nose, post-nasal drip, and congestion. Many in-office treatments for rhinitis are not comprehensive, while surgical options like septoplasty, turbinate reduction, or other ablative technologies yield mixed patient responses and may not address all symptoms.
The Aerin® Console and the RhinAer Stylus offer a compact and portable solution that addresses multiple contributors to chronic rhinitis, seamlessly fitting into any clinic, office, or hospital environment. the RhinAer procedure is a convenient and efficient treatment option to incorporate into your practice.
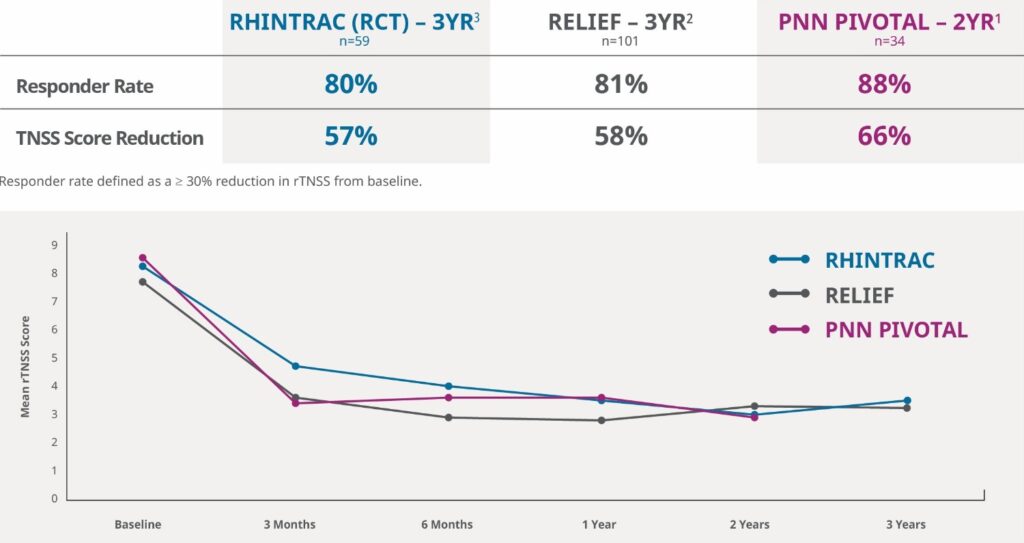
Proven Outcomes
RhinAer Stylus stands as the most studied non-invasive chronic rhinitis solution. It is distinguished by long-term clinical outcomes, including data on post-nasal drip and chronic cough. In a multicenter clinical study, RhinAer Stylus provided durable and significant relief of chronic rhinitis symptoms with an 88% responder rate out to two years.1
Demonstrated Effective, Long-Lasting Results
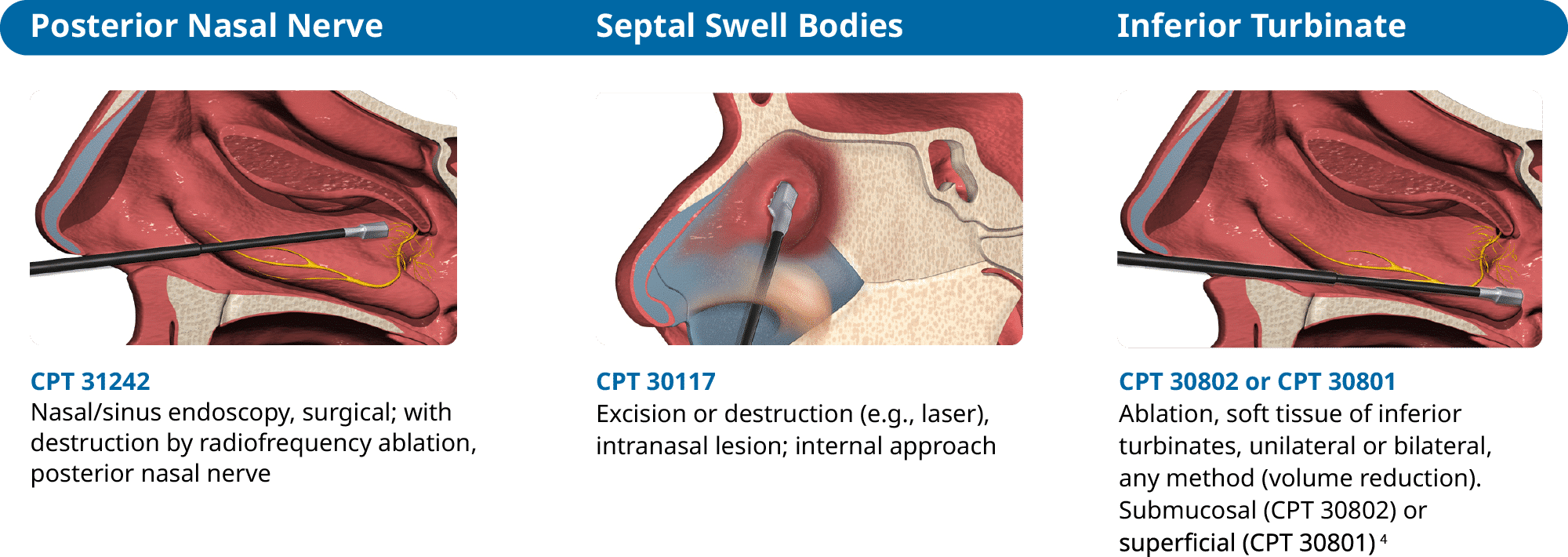
Increased Access for RhinAer Procedure Patients
RhinAer Stylus is covered by qualifying insurance plans when deemed medically necessary by a healthcare provider or ENT. The Aerin Reimbursement Center (ARC) is a HIPAA compliant, complimentary, third-party reimbursement support program made available by Aerin Medical to assist physicians and their patients with RhinAer Stylus insurance verification.
WEB1323-03.L
Disclaimer
The RhinAer® Stylus is indicated for use in otorhinolaryngology (ENT) surgery for the destruction of soft tissue in the nasal airway, including in posterior nasal nerve regions in patients with chronic rhinitis.
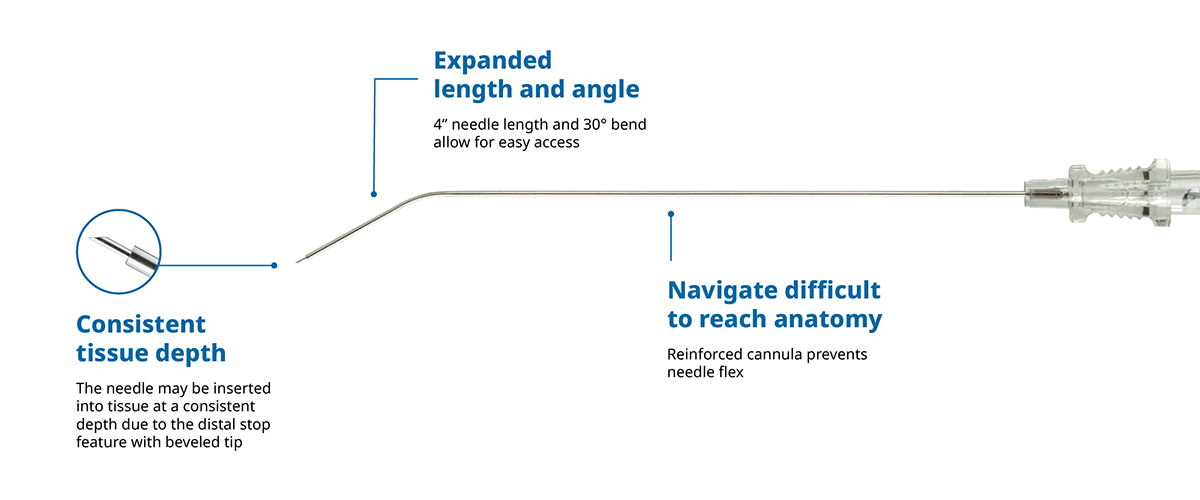
The Reach Needle™ is used for injecting local anesthetics into a patient to provide regional anesthesia.
Specific indications, contraindications, warnings, precautions, and safety information exist for these products. Aerin Medical relies on the physician to determine, assess and communicate to each patient all foreseeable risks of the procedure. A physician must always refer to the package insert, product label and/or instructions for use before using any of Aerin Medical’s products. Rx only.
Current Procedural Terminology (CPT) Copyright 2024 American Medical Association (AMA). All rights reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS Restrictions apply to government use. Fee schedules, relative value units, conversion factors, and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services.
The AMA assumes no liability for data contained or not contained herein. All trademarks are the property of their respective owners.
References:
- Ehmer D, McDuffie CM, McIntyre JB, Davis BM, Mehendale NH, Willis JH, Watkins JP, Kakarlapudi VV. Long-term Outcomes Following Temperature-Controlled Radiofrequency Neurolysis for the Treatment of Chronic Rhinitis. Allergy Rhinol (Providence). 2022 May 29;13:21526575221096045. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9158436/
- RELIEF STUDY: Lee JT, Abbas GM, Charous DD, et al. Three-Year Outcomes After Temperature-Controlled Radiofrequency Ablation of the Posterior Nasal Nerve for Chronic Rhinitis. American Journal of Rhinology & Allergy. 2025;0(0). doi:10.1177/19458924251360889
- RHINTRAC STUDY: Stolovitzky, J.P., Ow, R.A., Silvers, S.L., Tajudeen, B.A., McDuffie, C.M., Dean, M., Sedaghat, A.R., Phillips, K. and Takashima, M. (2025), 3-Year Outcomes of Temperature-Controlled Radiofrequency Ablation of the Posterior Nasal Nerve in Patients With Chronic Rhinitis. Int Forum Allergy Rhinol. e23577. https://doi.org/10.1002/alr.23577
- Provider to determine appropriate coding for destruction of turbinate either superficial or intramural (i.e., submucosal).